ISO 13485:2016 – Medical Devices:
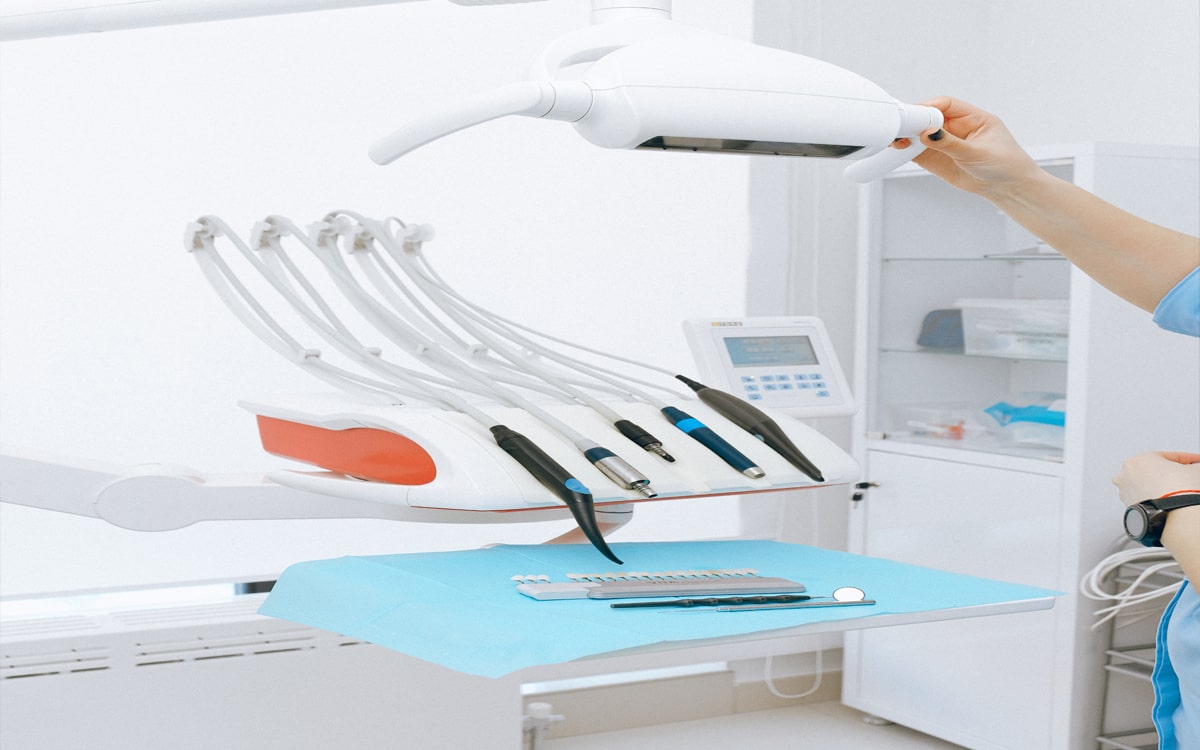
Safety and quality are non-negotiable in the medical devices industry because service lapse could impact human lives. In this regard, ISO 13485 establishes requirements for comprehensive quality management for the design and manufacturing of medical devices. It is a stand-alone QMS standard accepted as for ISO 9000 quality management standard series. It adapts the ISO 9000 process-based model for a regulated medical device manufacturing environment. The standard is more prescriptive in nature and requires a more thoroughly documented quality management system.
Why ISO 13485:2016 Certification?
ISO 13485 Certification can help an organization improve overall performance, enhance certainty, and expand market opportunities related to its processes and products. Organizations with this certification system demonstrate a commitment to quality for all stakeholders. Some of the benefits foreseen are:
- Improved company’s reputation as the certified organizations perceived as self-conscious and responsible for the quality of medical devices manufactured for its customers
- Improved and stronger decision making based on established facts & figures demonstrated safety of the produced medical devices.
- Better compliance with regulatory requirements and ensured customer satisfaction.
For more information email us This email address is being protected from spambots. You need JavaScript enabled to view it. Or contact us here